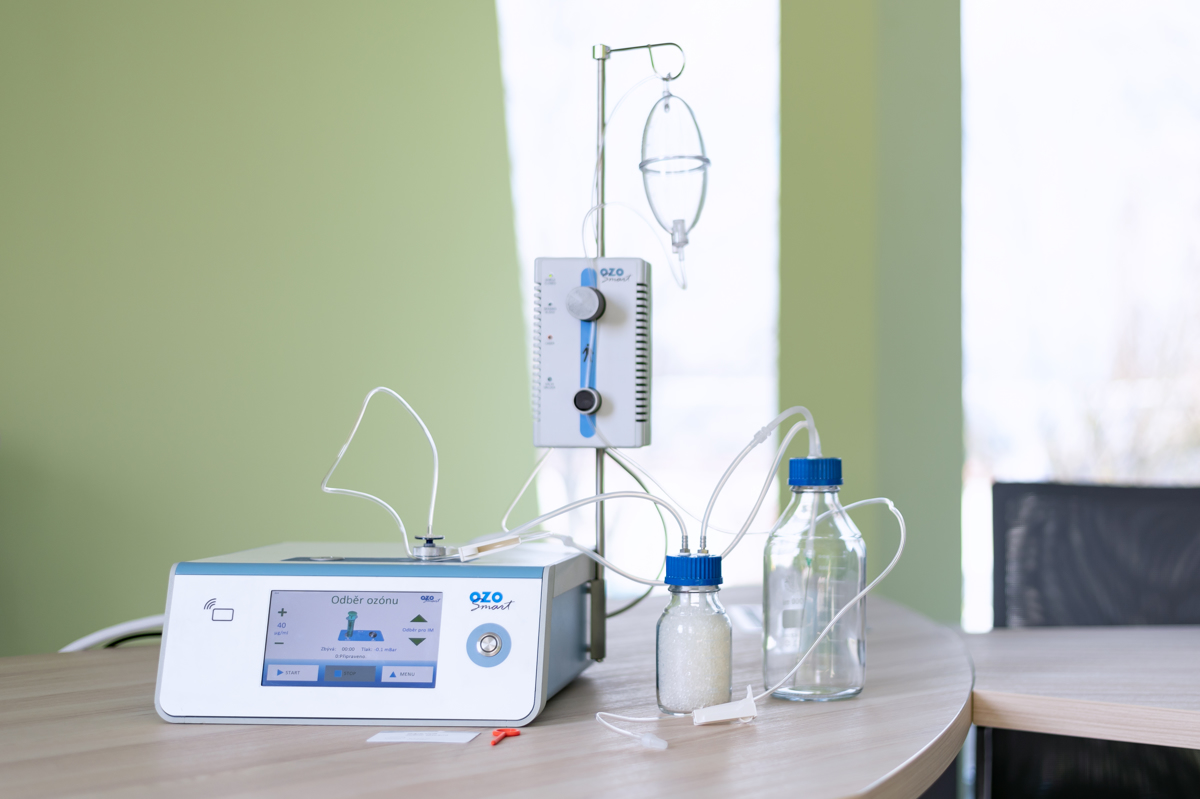
Certification of the Ozosmart medical device was co-financed from the EU call
ZAT is a long-established manufacturer of medical devices. As one of the first Czech companies operating in the healthcare sector, we have undergone a demanding certification process according to the new European legislation MDR (Medical Device Regulation), thanks to the EU co-financing from the call of Certification of medical devices and aerospace products and organizations under subcomponent 1.4.3 Reform No. 2: Support and Certification Group.
The certification focused on the Ozosmart therapeutic device for ozone therapy. It took the form of a multi-day audit, during which the audit teams examined all processes according to ČSN EN ISO 13 485, from development through manufacturing to sales, installation and service. The audit also included a review of all technical and clinical documentation related to the Ozosmart medical device.
Information on the Ozosmart medical device, which our company manufactures and distributes, is available on the ZAT website.
